Ammonia dehydrogenation
Over the last decades, hydrogen has gained considerable attention as an ideal and clean energy source. Its reaction with oxygen produces in fact only water as by-product and high efficiencies for energy conversion are achieved when hydrogen is employed as feedstock for power production in fuel cells.
However, its low energy density and the difficulties associated with gas handling are the main drawbacks associated to hydrogen which have so far prevented hydrogen-based technologies to achieve popularity for commercial application in the power production field. A solution to overcome these drawbacks consists in storing hydrogen in the chemical bonds of hydrogen carrier compounds.
Ammonia (NH3) is a particularly promising hydrogen carrier due to its high energy density, relatively low cost and ease of liquefaction, storage and transportation. Nevertheless, the use of ammonia as hydrogen carrier has so far been limited as ammonia decomposition into hydrogen and nitrogen is not efficiently practised yet on industrial scale. A conventional reactor system would require high temperatures for complete ammonia conversion, followed by a separation system to separate the pure hydrogen.
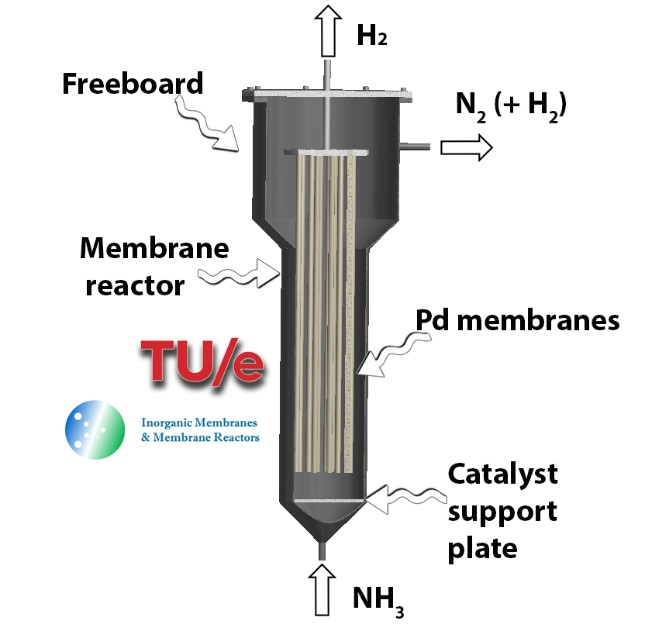
Within ARENHA project, a novel catalytic membrane reactor for ammonia decomposition will be developed at TUE. This advanced technology is expected to simultaneously perform ammonia decomposition and high-purity H2 separation within the same integrated unit. The reaction temperature required for the decomposition is thus lowered (with increase of efficiency) while the downstream separation is not required (decrease of capital costs). New materials and improved performance will enable a significant leap forward in comparison with state-of-the-art technology.
