Sorbents
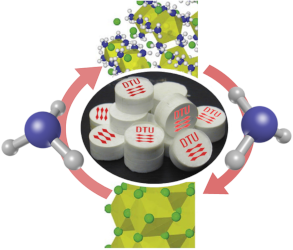
We aim at developing ammonia sorbents materials and beds 1) to increase the Haber-Bosch synthesis efficiency, reducing the operational parameters, 2) store in a safe and energy efficient manner the ammonia produced.
Haber-Bosch synthesis is typically conducted at 150–250 bar and 400-500 °C. The high reaction pressure shifts the reaction to the product side as direct result of Le Chatelier’s Principle: “A system at equilibrium, when subjected to a disturbance, responds to minimize the effect of the disturbance”. Instead of high pressure, we will shift the reaction to the product side by absorbing the synthesized ammonia as soon as produced. We will, for that purpose developed a sorbent material and a sorbent bed to comply with the new synthesis unit operated at relatively mild temperature and pressure conditions
Ammonia solid-State Storage. Storing and transporting ammonia are mainly done in one of two ways: in pressurized vessels at 16-25 bar, obtained by applying an over-pressure of inert gas or in insulated tanks at -33oC and ambient pressure. These two technologies raise security concerns, in case of leaks, and a non-negligible fraction of energy is used for cooling and refrigeration. Instead, solid ammonia storage in sorbents material offers a safer and more efficient alternative. We will, for that purpose developed a sorbent material and a sorbent tank design to be coupled with the new synthesis ammonia unit.
The two sorbents materials will necessarily be different because of the different working parameters of the two applications in terms of pressure and temperature. The materials will be a combination of different metal halides ammines, determined from a density functional theory screening study. The bed and reactor design will be developed from COMSOL multiphysics simulation
